Source: ivmmeta.com
Covid Analysis, (Version 36, Feb 24, 2021)



Introduction
We analyze all significant studies concerning the use of ivermectin for COVID-19. Search methods, inclusion criteria, effect extraction criteria (more serious outcomes have priority), all individual study data, PRISMA answers, and statistical methods are detailed in Appendix 1.
We present random effects meta-analysis results for all studies, for studies within each treatment stage, for mortality results only, for COVID-19 case results only, and for Randomized Controlled Trials (RCTs) only.
We also perform a simple analysis of the distribution of study effects. If treatment was not effective, the observed effects would be randomly distributed (or more likely to be negative if treatment is harmful).
We can compute the probability that the observed percentage of positive results (or higher) could occur due to chance with an ineffective treatment (the probability of >= k heads in n coin tosses, or the one-sided sign test / binomial test).
Analysis of publication bias is important and adjustments may be needed if there is a bias toward publishing positive results.Figure 2 shows stages of possible treatment for COVID-19.
Prophylaxis refers to regularly taking medication before becoming sick, in order to prevent or minimize infection.
Early Treatment refers to treatment immediately or soon after symptoms appear, while Late Treatment refers to more delayed treatment.

Results
Figure 3, 4, and 5 show results by treatment stage.
Figure 6, 7, 8, and 9 show forest plots for a random effects meta-analysis of all studies with pooled effects, and for studies reporting mortality results, COVID-19 case results, and viral clearance results only.
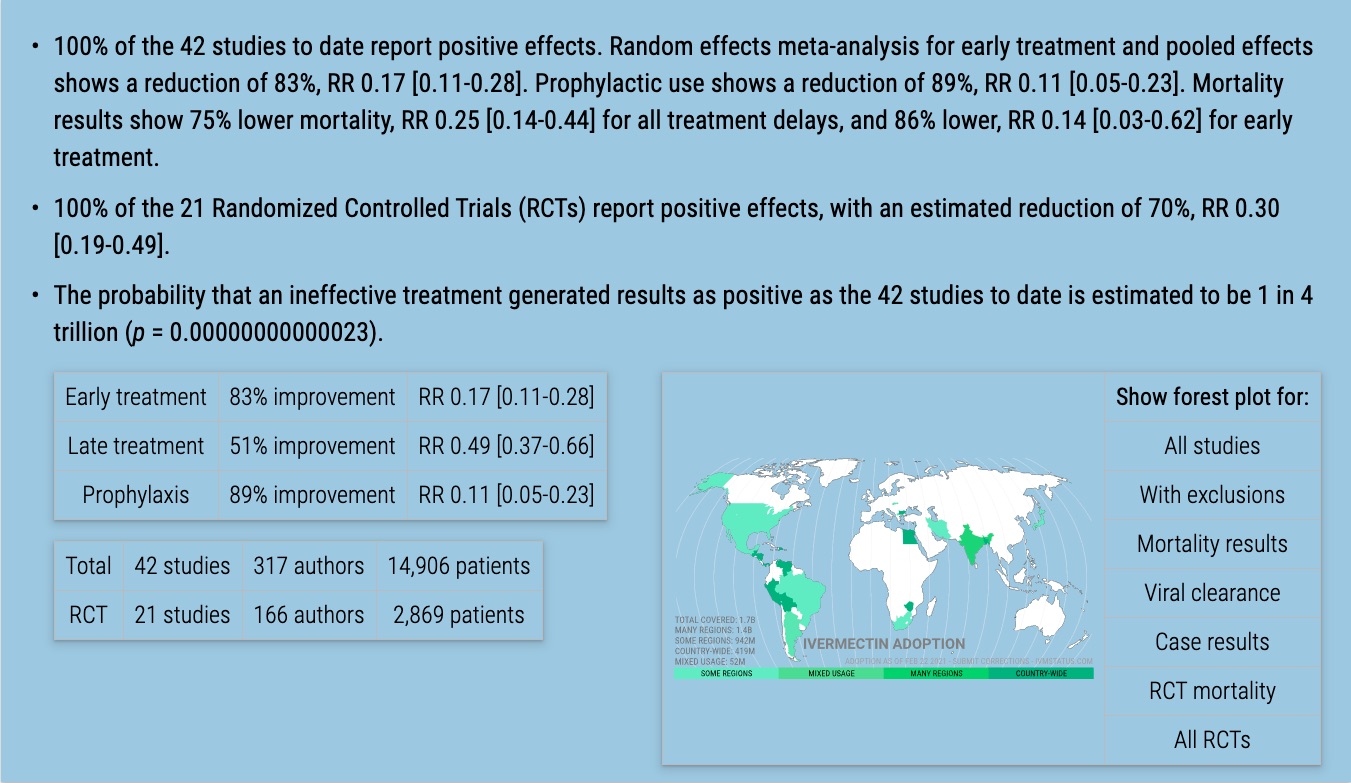
Table 1 summarizes the results.
| Treatment time | Number of studies reporting positive results | Total number of studies | Percentage of studies reporting positive results | Probability of an equal or greater percentage of positive results from an ineffective treatment | Random effects meta-analysis results |
| Early treatment | 13 | 13 | 100% | 0.00012 1 in 8 thousand | 83% improvement RR 0.17 [0.11‑0.28] p < 0.0001 |
| Late treatment | 18 | 18 | 100% | 0.0000038 1 in 262 thousand | 51% improvement RR 0.49 [0.37‑0.66] p < 0.0001 |
| Prophylaxis | 11 | 11 | 100% | 0.00049 1 in 2 thousand | 89% improvement RR 0.11 [0.05‑0.23] p < 0.0001 |
| All studies | 42 | 42 | 100% | 0.00000000000023 1 in 4 trillion | 75% improvement RR 0.25 [0.19‑0.34] p < 0.0001 |








Randomized Controlled Trials (RCTs)
Results restricted to Randomized Controlled Trials (RCTs) are shown in Figure 10, 11, 12, and 13, and Table 2. RCT results are similar to non-RCT results. Evidence shows that non-RCT trials can also provide reliable results. [Concato] find that well-designed observational studies do not systematically overestimate the magnitude of the effects of treatment compared to RCTs. [Anglemyer] summarized reviews comparing RCTs to observational studies and found little evidence for significant differences in effect estimates. [Lee] shows that only 14% of the guidelines of the Infectious Diseases Society of America were based on RCTs.
Evaluation of studies relies on an understanding of the study and potential biases. Limitations in an RCT can outweigh the benefits, for example excessive dosages, excessive treatment delays, or Internet survey bias could have a greater effect on results. Ethical issues may also prevent running RCTs for known effective treatments. For more on issues with RCTs see [Deaton, Nichol].






| Treatment time | Number of studies reporting positive results | Total number of studies | Percentage of studies reporting positive results | Probability of an equal or greater percentage of positive results from an ineffective treatment | Random effects meta-analysis results |
| Randomized Controlled Trials | 21 | 21 | 100% | 0.00000048 1 in 2 million | 70% improvement RR 0.30 [0.19‑0.49] p < 0.0001 |
| Randomized Controlled Trials (excluding late treatment) | 11 | 11 | 100% | 0.00049 1 in 2 thousand | 81% improvement RR 0.19 [0.11‑0.31] p < 0.0001 |
Exclusions
To avoid bias in the selection of studies, we include all studies in the main analysis. Here we show the results after excluding studies with critical issues likely to alter results, non-standard studies, and studies where very minimal detail is currently available.
[Soto-Becerra] is a database analysis covering anyone with ICD-10 COVID-19 codes, which includes asymptomatic PCR+ patients.
Therefore many patients in the control group are likely asymptomatic with regards to SARS-CoV-2, but in the hospital for another reason. For those that had symptomatic COVID-19, there is also likely significant confounding by indication.
KM curves show that the treatment groups were in more serious condition, with more than the total excess mortality at 30 days occurring on day 1. All treatments are worse than the control group at 30 days, while at the latest followup all treatments show lower mortality than control.
The machine learning system used also appears over-parameterized and likely to result in significant overfitting and inaccurate results. There is also no real control group in this study – patients receiving the treatments after 48 hours were put in the control group.
Authors also state that outcomes within 24 hours were excluded, however the KM curves show significant mortality at day 1 (only for the treatment groups).
Note that this study provides both 30 day mortality and weighted KM curves up to day 43 for ivermectin, we use the day 43 results as per our protocol.
There is no paper currently available for [Asghar].
[Vallejos] reports prophylaxis results, however only very minimal details are currently available in a news report. We include these results for additional confirmation of the efficacy observed in other trials, however this study is excluded here.
[Hellwig] provide an analysis of African countries and COVID-19 cases as a function of whether widespread prophylactic use of ivermectin is used for parasitic infections. Since this is a different kind of study to the typical trial, it is excluded here.
[Krolewiecki] show a concentration dependent antiviral activity of ivermectin whereby the viral decay rate for patients with ivermectin >160ng/mL was 0.64 log10 copies/reaction/day versus 0.13 for control. However, they do not provide the results for the entire treatment group vs. control.
Results for [Raad, Rezai] are available in [Hill], however no paper is currently available.Summarizing, the studies excluded are as follows, and the resulting forest plot is shown in Figure 14.
[Asghar], detail too minimal.
[Carvallo], control group formed from cases in the same hospital not in the study.
[Hellwig], not a typical trial, analysis of African countries that used or did not use ivermectin prophylaxis for parasitic infections.
[Raad], detail too minimal.
[Rezai], detail too minimal.
[Soto-Becerra], substantial unadjusted confounding by indication likely, includes PCR+ patients that may be asymptomatic for COVID-19 but in hospital for other reasons.
[Vallejos], detail too minimal.

Discussion
Publishing is often biased towards positive results, which we would need to adjust for when analyzing the percentage of positive results. For ivermectin, there is currently not enough data to evaluate publication bias with high confidence.
One method to evaluate bias is to compare prospective vs. retrospective studies. Prospective studies are likely to be published regardless of the result, while retrospective studies are more likely to exhibit bias.
For example, researchers may perform preliminary analysis with minimal effort and the results may influence their decision to continue. Retrospective studies also provide more opportunities for the specifics of data extraction and adjustments to influence results.
Figure 15 shows a scatter plot of results for prospective and retrospective studies. The median effect size for prospective studies is 82% improvement, compared to 78% for retrospective studies, i.e., currently the prospective studies, which are less likely to exhibit a positive publication bias, show more positive results.

Typical meta analyses involve subjective selection criteria, effect extraction rules, and study bias evaluation, which can be used to bias results towards a specific outcome. In order to avoid bias we include all studies and use a pre-specified method to extract results from all studies.
Every day that the approval of ivermectin is delayed results in thousands of deaths, so it is important to consider all available data.
We note that the positive results to date are very consistent and are relatively insensitive to potential selection criteria, effect extraction rules, and/or bias evaluation.
Studies vary significantly in terms of treatment delay, treatment regimen, patients characteristics, and (for the pooled effects analysis) outcomes, as reflected in the high degree of heterogeneity.
However the results consistently show a positive effect of treatment, and with the exception of some late treatment studies, the effect size is large.
Additional meta analyses confirming the effectiveness of ivermectin can be found in [Hill, Kory (B), Lawrie]. [Kory (B)] also review epidemiological data and provide suggested treatment regimens.
Conclusion
Ivermectin is an effective treatment for COVID-19. The probability that an ineffective treatment generated results as positive as the 42 studies to date is estimated to be 1 in 4 trillion (p = 0.00000000000023).
As expected for an effective treatment, early treatment is more successful, with an estimated reduction of 83% in the effect measured using a random effects meta-analysis, RR 0.17 [0.11-0.28].
Revisions
This paper is data driven, all graphs and numbers are dynamically generated. We will update the paper as new studies are released or with any corrections.
Please submit updates and corrections at the bottom of this page.
12/2: We added [Ahmed].
12/7: We added [Chaccour].
12/11: We added [Soto-Becerra].12/16: We added [Afsar].
12/17: We added [Alam].12/26: We added [Carvallo (C), Vallejos].
12/27: We added the total number of authors and patients.
12/29: We added meta analysis excluding late treatment.
12/31: We added additional details about the studies in the appendix.
1/2: We added dosage information and we added the number of patients to the forest plots.
1/5: We added direct links to the study details in the forest plots.
1/6: We added [Babalola].
1/7: We added direct links to the study details in the chronological plots.
1/9: We added [Kirti]. Due to the much larger size of the control group in [Bernigaud], we limited the size of the control group to be the same as the treatment group for calculation of the number of patients.
1/10: We put all prophylaxis studies in a single group.
1/11: We added [Chala].
1/12: We added [Okumuş].
1/15: We added the effect measured for each study in the forest plots.
1/16: We moved the analysis with exclusions to the main text, and added additional commentary.
1/17: We added [Asghar].
1/19: We added [Raad, Rezai]. [Chaccour] was updated to the journal version of the paper.
1/25: We updated [Vallejos] with the recently released results.
1/26: We updated [Shouman] with the journal version of the article.
2/2: We added [Mohan].
2/5: We added [Bukhari].
2/10: We added [Lima-Morales].
2/11: We added more details on the analysis of prospective vs. retrospective studies.
2/12: We added [Schwartz].
2/14: We added analysis restricted to COVID-19 case outcomes, and we added additional results in the abstract.
2/15: We added [Behera].
2/16: We updated [Behera (B)] to the journal version of the paper.
2/17: We added [Elalfy], and we added analysis restricted to viral clearance outcomes, and mortality results restricted to RCTs.
2/18: We updated [Babalola] to the journal version of the paper.
2/23: We added [Beltran-Gonzalez].
2/24: We added a comparison of the evidence base and WHO approval status for the use of ivermectin with scabies and COVID-19. We updated [Okumuş] with the Research Square preprint.
Appendix 1. Methods and Study Results
We performed ongoing searches of PubMed, medRxiv, ClinicalTrials.gov, The Cochrane Library, Google Scholar, Collabovid, Research Square, ScienceDirect, Oxford University Press, the reference lists of other studies and meta-analyses, and submissions to the site c19ivermectin.com, which regularly receives submissions of studies upon publication.
Search terms were ivermectin and COVID-19 or SARS-CoV-2, or simply ivermectin.
Automated searches are performed every hour with notifications of new matches.
All studies regarding the use of ivermectin for COVID-19 that report an effect compared to a control group are included in the main analysis.
This is a living analysis and is updated regularly.
We extracted effect sizes and associated data from all studies. If studies report multiple kinds of effects then the most serious outcome is used in calculations for that study.
For example, if effects for mortality and cases are both reported, the effect for mortality is used, this may be different to the effect that a study focused on.
If symptomatic results are reported at multiple times, we used the latest time, for example if mortality results are provided at 14 days and 28 days, the results at 28 days are used.
Mortality alone is preferred over combined outcomes. Outcomes with zero events in both arms were not used. Clinical outcome is considered more important than PCR testing status.
For PCR results reported at multiple times, where a majority of patients recover in both groups, preference is given to results mid-recovery (after most or all patients have recovered there is no room for an effective treatment to do better).
When results provide an odds ratio, we computed the relative risk when possible, or converted to a relative risk according to [Zhang].
Reported confidence intervals and p-values were used when available, using adjusted values when provided. If multiple types of adjustments are reported including propensity score matching (PSM), the PSM results are used.
When needed, conversion between reported p-values and confidence intervals followed [Altman, Altman (B)], and Fisher’s exact test was used to calculate p-values for event data. If continuity correction for zero values is required, we use the reciprocal of the opposite arm with the sum of the correction factors equal to 1 [Sweeting].
Results are all expressed with RR < 1.0 suggesting effectiveness. Most results are the relative risk of something negative. If studies report relative times, results are expressed as the ratio of the time for the ivermectin group versus the time for the control group.
Calculations are done in Python (3.9.1) with scipy (1.5.4), pythonmeta (1.11), numpy (1.19.4), statsmodels (0.12.1), and plotly (4.14.1).The forest plots are computed using PythonMeta [Deng] with the DerSimonian and Laird random effects model (the fixed effect assumption is not plausible in this case).
The forest plots show simplified dosages for comparison, these are the total dose in the first two days for treatment, and the monthly dose for prophylaxis, for a 70kg person.
For full dosage details see below.
We received no funding, this research is done in our spare time. We have no affiliations with any pharmaceutical companies or political parties.
We have classified studies as early treatment if most patients are not already at a severe stage at the time of treatment, and treatment started within 5 days after the onset of symptoms, although a shorter time may be preferable.
Antivirals are typically only considered effective when used within a shorter timeframe, for example 0-36 or 0-48 hours for oseltamivir, with longer delays not being effective [McLean, Treanor].
Due to the much larger size of the control group in [Bernigaud], we limit the size of the control group to be the same as the treatment group for calculation of the number of patients.
A summary of study results is below.
Please submit updates and corrections at the bottom of this page.
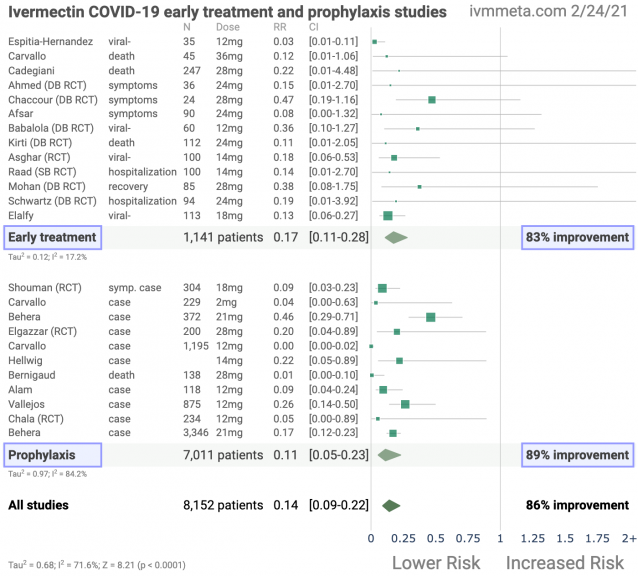
Early treatment
Effect extraction follows pre-specified rules as detailed above and gives priority to more serious outcomes. Only the first (most serious) outcome is used in calculations, which may differ from the effect a paper focuses on.
| [Afsar], 12/15/2020, retrospective, Pakistan, South Asia, preprint, 6 authors, dosage 12mg days 1-6. Submit Corrections or Updates. | risk of fever at day 14, 92.2% lower, RR 0.08, p = 0.04, treatment 0 of 37 (0.0%), control 7 of 53 (13.2%). |
| [Ahmed], 12/2/2020, Double Blind Randomized Controlled Trial, Bangladesh, South Asia, peer-reviewed, mean age 42.0, 15 authors, dosage 12mg days 1-5, ivermectin + doxycycline group took only a single dose of ivermectin. Submit Corrections or Updates. | risk of unresolved symptoms, 85.0% lower, RR 0.15, p = 0.09, treatment 0 of 17 (0.0%), control 3 of 19 (15.8%), day 7 fever ivermectin. |
| risk of unresolved symptoms, 62.7% lower, RR 0.37, p = 0.35, treatment 1 of 17 (5.9%), control 3 of 19 (15.8%), day 7 fever ivermectin + doxycycline. | |
| risk of no virological cure, 42.5% lower, RR 0.58, p = 0.01, treatment 11 of 22 (50.0%), control 20 of 23 (87.0%), day 7 ivermectin. | |
| risk of no virological cure, 20.0% lower, RR 0.80, p = 0.28, treatment 16 of 23 (69.6%), control 20 of 23 (87.0%), day 7 ivermectin + doxycycline. | |
| risk of no virological cure, 62.7% lower, RR 0.37, p = 0.02, treatment 5 of 22 (22.7%), control 14 of 23 (60.9%), day 14 ivermectin. | |
| risk of no virological cure, 35.7% lower, RR 0.64, p = 0.24, treatment 9 of 23 (39.1%), control 14 of 23 (60.9%), day 14 ivermectin + doxycycline. | |
| time to viral-, 23.6% lower, relative time 0.76, p = 0.02, ivermectin. | |
| time to viral-, 9.4% lower, relative time 0.91, p = 0.27, ivermectin + doxycycline. | |
| [Asghar], 1/16/2021, Randomized Controlled Trial, Pakistan, South Asia, preprint, 1 author, dosage 200μg/kg days 1, 8. Submit Corrections or Updates. | risk of no virological cure, 82.1% lower, RR 0.18, p < 0.001, treatment 50, control 50, day 7. |
| [Babalola], 1/6/2021, Double Blind Randomized Controlled Trial, Nigeria, Africa, peer-reviewed, baseline oxygen requirements 8.3%, 10 authors, dosage 12mg or 6mg q84h for two weeks. Submit Corrections or Updates. | adjusted risk of viral+ at day 5, 63.9% lower, RR 0.36, p = 0.11, treatment 40, control 20, adjusted per study. |
| risk of no virological cure, 58.0% lower, RR 0.42, p = 0.01, treatment 20, control 20, 12mg – Cox proportional hazard model. | |
| risk of no virological cure, 40.5% lower, RR 0.60, p = 0.12, treatment 20, control 20, 6mg – Cox proportional hazard model. | |
| time to viral-, 49.2% lower, relative time 0.51, treatment 20, control 20, 12mg. | |
| time to viral-, 34.4% lower, relative time 0.66, treatment 20, control 20, 6mg. | |
| [Cadegiani], 11/4/2020, prospective, Brazil, South America, preprint, 4 authors, dosage 200μg/kg days 1-3. Submit Corrections or Updates. | risk of death, 78.3% lower, RR 0.22, p = 0.50, treatment 0 of 110 (0.0%), control 2 of 137 (1.5%), control group 1. |
| risk of ventilation, 94.2% lower, RR 0.06, p = 0.005, treatment 0 of 110 (0.0%), control 9 of 137 (6.6%), control group 1. | |
| risk of hospitalization, 98.0% lower, RR 0.02, p < 0.001, treatment 0 of 110 (0.0%), control 27 of 137 (19.7%), control group 1. | |
| [Carvallo], 9/15/2020, prospective, Argentina, South America, preprint, mean age 55.7, 3 authors, dosage 36mg days 1, 8, dose varied depending on patient condition – mild 24mg, moderate 36mg, severe 48mg. Submit Corrections or Updates. | risk of death for hospitalized cases in study vs. cases in the same hospital not in the study, 87.9% lower, RR 0.12, p = 0.05, treatment 1 of 33 (3.0%), control 3 of 12 (25.0%), the only treatment death was a patient already in the ICU before treatment. |
| [Chaccour], 12/7/2020, Double Blind Randomized Controlled Trial, Spain, Europe, peer-reviewed, 23 authors, dosage 400μg/kg single dose. Submit Corrections or Updates. | risk of unresolved symptoms, 52.9% lower, RR 0.47, p < 0.05, treatment 12, control 12, relative probability of symptoms at day 28. |
| viral load, 94.6% lower, relative load 0.05, treatment 12, control 12, day 7 mid-recovery. | |
| [Elalfy], 2/16/2021, retrospective, Egypt, Middle East, peer-reviewed, 15 authors, dosage 18mg days 1, 4, 7, 10, 13, <90kg 18mg, 90-120kg 24mg, >120kg 30mg. Submit Corrections or Updates. | risk of no virological cure, 86.9% lower, RR 0.13, p < 0.001, treatment 7 of 62 (11.3%), control 44 of 51 (86.3%), day 15. |
| risk of no virological cure, 58.1% lower, RR 0.42, p < 0.001, treatment 26 of 62 (41.9%), control 51 of 51 (100.0%), day 7. | |
| [Espitia-Hernandez], 8/15/2020, retrospective, Mexico, North America, peer-reviewed, mean age 45.1, 5 authors, dosage 6mg days 1-2, 8-9. Submit Corrections or Updates. | risk of viral+ at day 10, 97.2% lower, RR 0.03, p < 0.001, treatment 0 of 28 (0.0%), control 7 of 7 (100.0%). |
| [Kirti], 1/9/2021, Double Blind Randomized Controlled Trial, India, South Asia, preprint, 11 authors, dosage 12mg days 1, 2. Submit Corrections or Updates. | risk of death, 88.7% lower, RR 0.11, p = 0.12, treatment 0 of 55 (0.0%), control 4 of 57 (7.0%). |
| risk of ventilation, 79.3% lower, RR 0.21, p = 0.09, treatment 1 of 55 (1.8%), control 5 of 57 (8.8%). | |
| risk of ICU admission, 13.6% lower, RR 0.86, p = 0.80, treatment 5 of 55 (9.1%), control 6 of 57 (10.5%). | |
| risk of no virological cure, 11.6% higher, RR 1.12, p = 0.35, treatment 42 of 55 (76.4%), control 39 of 57 (68.4%). | |
| [Mohan], 2/2/2021, Double Blind Randomized Controlled Trial, India, South Asia, preprint, 27 authors, dosage 400μg/kg single dose, 200μg/kg also tested. Submit Corrections or Updates. | risk of no discharge at day 14, 62.5% lower, RR 0.38, p = 0.27, treatment 2 of 40 (5.0%), control 6 of 45 (13.3%), ivermectin 24mg. |
| risk of no discharge at day 14, 43.8% lower, RR 0.56, p = 0.49, treatment 3 of 40 (7.5%), control 6 of 45 (13.3%), ivermectin 12mg. | |
| risk of no virological cure, 10.3% lower, RR 0.90, p = 0.65, treatment 20 of 36 (55.6%), control 26 of 42 (61.9%), ivermectin 24mg, day 7. | |
| risk of no virological cure, 3.2% higher, RR 1.03, p = 1.00, treatment 23 of 36 (63.9%), control 26 of 42 (61.9%), ivermectin 12mg, day 7. | |
| risk of no virological cure, 23.8% lower, RR 0.76, p = 0.18, treatment 21 of 40 (52.5%), control 31 of 45 (68.9%), ivermectin 24mg, day 5. | |
| risk of no virological cure, 5.6% lower, RR 0.94, p = 0.82, treatment 26 of 40 (65.0%), control 31 of 45 (68.9%), ivermectin 12mg, day 5. | |
| [Raad], 1/16/2021, Single Blind Randomized Controlled Trial, Lebanon, Middle East, preprint, 1 author, dosage 200μg/kg single dose. Submit Corrections or Updates. | risk of hospitalization, 85.7% lower, RR 0.14, p = 0.24, treatment 0 of 50 (0.0%), control 3 of 50 (6.0%). |
| risk of viral load, 59.0% lower, RR 0.41, p = 0.01, treatment 50, control 50, percentage relative improvement in Ct value with treatment at day 3. | |
| [Schwartz], 2/12/2021, Double Blind Randomized Controlled Trial, Israel, Middle East, preprint, 1 author, dosage 12mg days 1-3, 15mg for patients >= 70kg. Submit Corrections or Updates. | risk of hospitalization, 80.7% lower, RR 0.19, p = 0.23, treatment 0 of 49 (0.0%), control 2 of 45 (4.4%). |
| risk of no virological cure, 51.4% lower, RR 0.49, p = 0.01, treatment 16 of 49 (32.7%), control 25 of 45 (55.6%), adjusted per study, odds ratio converted to relative risk, multivariable logistic regression, day 6, Ct>30. | |
| risk of no virological cure, 54.1% lower, RR 0.46, p = 0.02, treatment 9 of 49 (18.4%), control 18 of 45 (40.0%), day 10, Ct>30. | |
| risk of no virological cure, 54.1% lower, RR 0.46, p = 0.02, treatment 10 of 49 (20.4%), control 20 of 45 (44.4%), day 8, Ct>30. | |
| risk of no virological cure, 41.2% lower, RR 0.59, p = 0.04, treatment 16 of 49 (32.7%), control 25 of 45 (55.6%), day 6, Ct>30. | |
| risk of no virological cure, 37.9% lower, RR 0.62, p = 0.09, treatment 11 of 26 (42.3%), control 15 of 22 (68.2%), day 4, Ct>30. |
Late treatment
Effect extraction follows pre-specified rules as detailed above and gives priority to more serious outcomes. Only the first (most serious) outcome is used in calculations, which may differ from the effect a paper focuses on.
| [Beltran-Gonzalez], 2/23/2021, Double Blind Randomized Controlled Trial, Mexico, North America, peer-reviewed, mean age 53.8, 13 authors, dosage 12mg single dose, 18mg for patients >80kg. Submit Corrections or Updates. | risk of death, 14.4% lower, RR 0.86, p = 1.00, treatment 5 of 36 (13.9%), control 6 of 37 (16.2%). |
| risk of respiratory deterioration or death, 8.6% lower, RR 0.91, p = 1.00, treatment 8 of 36 (22.2%), control 9 of 37 (24.3%). | |
| risk of no hospital discharge, 37.0% higher, RR 1.37, p = 0.71, treatment 4 of 36 (11.1%), control 3 of 37 (8.1%). | |
| [Budhiraja], 11/18/2020, retrospective, India, South Asia, preprint, 12 authors, dosage not specified. Submit Corrections or Updates. | risk of death, 99.1% lower, RR 0.009, p = 0.04, treatment 0 of 34 (0.0%), control 103 of 942 (10.9%). |
| [Bukhari], 2/5/2021, Randomized Controlled Trial, Pakistan, Middle East, preprint, 10 authors, dosage 12mg single dose. Submit Corrections or Updates. | risk of no virological cure, 82.4% lower, RR 0.18, p < 0.001, treatment 4 of 41 (9.8%), control 25 of 45 (55.6%), day 7. |
| risk of no virological cure, 38.7% lower, RR 0.61, p < 0.001, treatment 24 of 41 (58.5%), control 43 of 45 (95.6%), day 3. | |
| [Camprubí], 11/11/2020, retrospective, Spain, Europe, peer-reviewed, 9 authors, dosage 200μg/kg single dose. Submit Corrections or Updates. | risk of ICU admission, 33.3% lower, RR 0.67, p = 1.00, treatment 2 of 13 (15.4%), control 3 of 13 (23.1%), ICU at day 8. |
| risk of no improvement at day 8, 33.3% higher, RR 1.33, p = 1.00, treatment 4 of 13 (30.8%), control 3 of 13 (23.1%). | |
| [Chachar], 9/30/2020, Randomized Controlled Trial, India, South Asia, peer-reviewed, 6 authors, dosage 36mg, 12mg stat, 12mg after 12 hours, 12mg after 24 hours. Submit Corrections or Updates. | risk of no recovery at day 7, 10.0% lower, RR 0.90, p = 0.50, treatment 9 of 25 (36.0%), control 10 of 25 (40.0%). |
| [Elgazzar], 11/13/2020, Randomized Controlled Trial, Egypt, Africa, preprint, 6 authors, dosage 400μg/kg days 1-4. Submit Corrections or Updates. | risk of death, 91.7% lower, RR 0.08, p < 0.001, treatment 2 of 200 (1.0%), control 24 of 200 (12.0%). |
| risk of death, 88.9% lower, RR 0.11, p = 0.12, treatment 0 of 100 (0.0%), control 4 of 100 (4.0%), mild/moderate COVID-19. | |
| risk of death, 90.0% lower, RR 0.10, p < 0.001, treatment 2 of 100 (2.0%), control 20 of 100 (20.0%), severe COVID-19. | |
| [Gorial], 7/8/2020, retrospective, Iraq, Middle East, preprint, 9 authors, dosage 200μg/kg single dose. Submit Corrections or Updates. | risk of death, 71.0% lower, RR 0.29, p = 1.00, treatment 0 of 16 (0.0%), control 2 of 71 (2.8%). |
| hospitalization time, 42.0% lower, relative time 0.58, p < 0.001, treatment 16, control 71. | |
| [Hashim], 10/26/2020, Single Blind Randomized Controlled Trial, Iraq, Middle East, preprint, 6 authors, dosage 200μg/kg days 1-2, some patients received a third dose on day 8. Submit Corrections or Updates. | risk of death, 66.7% lower, RR 0.33, p = 0.27, treatment 2 of 70 (2.9%), control 6 of 70 (8.6%), all patients. |
| risk of death, 91.7% lower, RR 0.08, p = 0.03, treatment 0 of 59 (0.0%), control 6 of 70 (8.6%), excluding critical patients. | |
| [Khan], 9/24/2020, retrospective, Bangladesh, South Asia, preprint, median age 35.0, 8 authors, dosage 12mg single dose. Submit Corrections or Updates. | risk of death, 87.0% lower, RR 0.13, p < 0.05, treatment 1 of 115 (0.9%), control 9 of 133 (6.8%). |
| time to viral-, 73.3% lower, relative time 0.27, p < 0.001, treatment 115, control 133. | |
| [Lima-Morales], 2/10/2021, prospective, Mexico, North America, peer-reviewed, 9 authors, dosage 12mg single dose. Submit Corrections or Updates. | risk of death, 77.7% lower, RR 0.22, p < 0.001, treatment 15 of 481 (3.1%), control 52 of 287 (18.1%), adjusted per study, odds ratio converted to relative risk, multivariate. |
| risk of hospitalization, 67.4% lower, RR 0.33, p < 0.001, treatment 44 of 481 (9.1%), control 89 of 287 (31.0%), adjusted per study, odds ratio converted to relative risk, multivariate. | |
| risk of no recovery, 58.6% lower, RR 0.41, p < 0.001, treatment 75 of 481 (15.6%), control 118 of 287 (41.1%), adjusted per study, odds ratio converted to relative risk, recovery at day 14 after symptoms, multivariate. | |
| [Mahmud], 10/9/2020, Double Blind Randomized Controlled Trial, Bangladesh, South Asia, preprint, 1 author, dosage 12mg single dose. Submit Corrections or Updates. | risk of death, 85.7% lower, RR 0.14, p = 0.25, treatment 0 of 183 (0.0%), control 3 of 183 (1.6%). |
| risk of no recovery, 49.0% lower, RR 0.51, p < 0.004, treatment 42 of 183 (23.0%), control 67 of 180 (37.2%), adjusted per study. | |
| risk of disease progression, 55.0% lower, RR 0.45, p < 0.01, treatment 16 of 183 (8.7%), control 32 of 180 (17.8%), adjusted per study. | |
| risk of no virological cure, 42.0% lower, RR 0.58, p < 0.001, treatment 14 of 183 (7.7%), control 36 of 180 (20.0%), adjusted per study. | |
| [Niaee], 11/24/2020, Double Blind Randomized Controlled Trial, Iran, Middle East, preprint, mean age 56.0, 14 authors, dosage 400μg/kg single dose, dose varies in different groups. Submit Corrections or Updates. | risk of death, 81.8% lower, RR 0.18, p = 0.001, treatment 4 of 120 (3.3%), control 11 of 60 (18.3%), All IVM vs. all control. |
| risk of death, 94.3% lower, RR 0.06, p = 0.01, treatment 0 of 30 (0.0%), control 11 of 60 (18.3%), IVM single dose 200mcg/kg vs. all control. | |
| risk of death, 45.5% lower, RR 0.55, p = 0.37, treatment 3 of 30 (10.0%), control 11 of 60 (18.3%), IVM three dose 200mcg/kg vs. all control. | |
| risk of death, 94.3% lower, RR 0.06, p = 0.01, treatment 0 of 30 (0.0%), control 11 of 60 (18.3%), IVM single dose 400mcg/kg vs. all control. | |
| risk of death, 81.8% lower, RR 0.18, p = 0.06, treatment 1 of 30 (3.3%), control 11 of 60 (18.3%), IVM three dose 400/200/200mcg/kg vs. all control. | |
| [Okumuş], 1/12/2021, Double Blind Randomized Controlled Trial, Turkey, Middle East, preprint, 15 authors, dosage 200μg/kg days 1-5, 36-50kg – 9mg, 51-65kg – 12mg, 66-79kg – 15mg, >80kg 200μg/kg. Submit Corrections or Updates. | risk of death, 33.3% lower, RR 0.67, p = 0.55, treatment 6 of 30 (20.0%), control 9 of 30 (30.0%). |
| risk of no improvement at day 10, 42.9% lower, RR 0.57, p = 0.18, treatment 8 of 30 (26.7%), control 14 of 30 (46.7%). | |
| risk of no improvement at day 5, 15.8% lower, RR 0.84, p = 0.60, treatment 16 of 30 (53.3%), control 19 of 30 (63.3%). | |
| risk of no virological cure, 80.0% lower, RR 0.20, p = 0.02, treatment 2 of 16 (12.5%), control 5 of 8 (62.5%), day 10. | |
| [Podder], 9/3/2020, Randomized Controlled Trial, Bangladesh, South Asia, peer-reviewed, 4 authors, dosage 200μg/kg single dose. Submit Corrections or Updates. | recovery time from enrollment, 16.1% lower, relative time 0.84, p = 0.34, treatment 32, control 30. |
| [Rajter], 10/13/2020, retrospective, USA, North America, peer-reviewed, 6 authors, dosage 200μg/kg single dose. Submit Corrections or Updates. | risk of death, 46.0% lower, RR 0.54, p = 0.04, treatment 13 of 98 (13.3%), control 24 of 98 (24.5%), adjusted per study, odds ratio converted to relative risk, PSM. |
| risk of death, 66.9% lower, RR 0.33, p = 0.03, treatment 26 of 173 (15.0%), control 27 of 107 (25.2%), adjusted per study, odds ratio converted to relative risk, multivariate. | |
| [Rezai], 1/19/2021, Randomized Controlled Trial, Iran, Middle East, preprint, 1 author, dosage 200μg/kg single dose. Submit Corrections or Updates. | recovery time, 21.2% lower, relative time 0.79, p = 0.02, treatment 51, control 52. |
| hospitalization time, 17.9% lower, relative time 0.82, p = 0.01, treatment 51, control 52. | |
| [Soto-Becerra], 10/8/2020, retrospective, database analysis, Peru, South America, preprint, median age 59.4, 4 authors, dosage 200μg/kg single dose. Submit Corrections or Updates. | risk of death, 17.1% lower, RR 0.83, p = 0.01, treatment 92 of 203 (45.3%), control 1438 of 2630 (54.7%), IVM vs. control day 43 (last day available) weighted KM from figure 3, per the pre-specified rules, the last available day mortality results have priority. |
| risk of death, 39.0% higher, RR 1.39, p = 0.16, treatment 47 of 203 (23.2%), control 401 of 2630 (15.2%), adjusted per study, day 30, Table 2, IVM wHR. | |
| [Spoorthi], 11/14/2020, prospective, India, South Asia, peer-reviewed, 2 authors, dosage not specified. Submit Corrections or Updates. | recovery time, 21.1% lower, relative time 0.79, p = 0.03, treatment 50, control 50. |
| hospitalization time, 15.5% lower, relative time 0.84, p = 0.01, treatment 50, control 50. |
Prophylaxis
Effect extraction follows pre-specified rules as detailed above and gives priority to more serious outcomes. Only the first (most serious) outcome is used in calculations, which may differ from the effect a paper focuses on.
| [Alam], 12/15/2020, prospective, Bangladesh, South Asia, peer-reviewed, 13 authors, dosage 12mg monthly. Submit Corrections or Updates. | risk of COVID-19 case, 90.6% lower, RR 0.09, p < 0.001, treatment 4 of 58 (6.9%), control 44 of 60 (73.3%). |
| [Behera], 2/15/2021, prospective, India, South Asia, preprint, 13 authors, dosage 300μg/kg days 1, 4. Submit Corrections or Updates. | risk of COVID-19 case, 83.0% lower, RR 0.17, p < 0.001, treatment 2199, control 1147, two doses. |
| risk of COVID-19 case, 4.0% higher, RR 1.04, p = 0.85, treatment 186, control 1147, patients only receiving the first dose. | |
| [Behera (B)], 11/3/2020, retrospective, India, South Asia, peer-reviewed, 13 authors, dosage 300μg/kg days 1, 4. Submit Corrections or Updates. | risk of COVID-19 case, 53.8% lower, RR 0.46, p < 0.001, treatment 41 of 117 (35.0%), control 145 of 255 (56.9%), adjusted per study, odds ratio converted to relative risk, model 2 2+ doses conditional logistic regression. |
| risk of COVID-19 case, 44.5% lower, RR 0.56, p < 0.001, treatment 41 of 117 (35.0%), control 145 of 255 (56.9%), odds ratio converted to relative risk, matched pair analysis. | |
| [Bernigaud], 11/28/2020, retrospective, France, Europe, peer-reviewed, 12 authors, dosage 200μg/kg days 1, 8, 15, 400μg/kg days 1, 8, 15, two different dosages. Submit Corrections or Updates. | risk of death, 99.4% lower, RR 0.006, p = 0.08, treatment 0 of 69 (0.0%), control 150 of 3062 (4.9%). |
| risk of COVID-19 case, 55.1% lower, RR 0.45, p = 0.01, treatment 7 of 69 (10.1%), control 692 of 3062 (22.6%). | |
| [Carvallo (B)], 11/17/2020, prospective, Argentina, South America, peer-reviewed, 4 authors, dosage 12mg weekly. Submit Corrections or Updates. | risk of COVID-19 case, 99.9% lower, RR 0.001, p < 0.001, treatment 0 of 788 (0.0%), control 237 of 407 (58.2%). |
| [Carvallo (C)], 10/19/2020, prospective, Argentina, South America, preprint, 1 author, dosage 1mg days 1-14. Submit Corrections or Updates. | risk of COVID-19 case, 96.3% lower, RR 0.04, p < 0.001, treatment 0 of 131 (0.0%), control 11 of 98 (11.2%). |
| [Chahla], 1/11/2021, Randomized Controlled Trial, Argentina, South America, preprint, 1 author, dosage 12mg weekly. Submit Corrections or Updates. | risk of COVID-19 case, 94.7% lower, RR 0.05, p = 0.003, treatment 0 of 117 (0.0%), control 9 of 117 (7.7%), moderate/severe COVID-19. |
| risk of COVID-19 case, 84.0% lower, RR 0.16, p < 0.001, treatment 4 of 117 (3.4%), control 25 of 117 (21.4%), all cases. | |
| [Elgazzar (B)], 11/13/2020, Randomized Controlled Trial, Egypt, Africa, preprint, 6 authors, dosage 400μg/kg weekly. Submit Corrections or Updates. | risk of COVID-19 case, 80.0% lower, RR 0.20, p = 0.03, treatment 2 of 100 (2.0%), control 10 of 100 (10.0%). |
| [Hellwig], 11/28/2020, retrospective, multiple countries, Africa, peer-reviewed, 2 authors, dosage 200μg/kg, dose varied, typically 150-200μg/kg. Submit Corrections or Updates. | risk of COVID-19 case, 78.0% lower, RR 0.22, p < 0.02, African countries. |
| risk of COVID-19 case, 80.0% lower, RR 0.20, p < 0.001, worldwide. | |
| [Shouman], 8/28/2020, Randomized Controlled Trial, Egypt, Africa, peer-reviewed, 8 authors, dosage 18mg days 1, 3, dose varies depending on weight – 40-60kg: 15mg, 60-80kg: 18mg, >80kg: 24mg. Submit Corrections or Updates. | risk of symptomatic case, 91.3% lower, RR 0.09, p < 0.001, treatment 15 of 203 (7.4%), control 59 of 101 (58.4%), adjusted per study, multivariate. |
| risk of COVID-19 severe case, 92.9% lower, RR 0.07, p = 0.002, treatment 1 of 203 (0.5%), control 7 of 101 (6.9%), unadjusted. | |
| [Vallejos], 12/20/2020, retrospective, Argentina, South America, preprint, 1 author, dosage 12mg weekly. Submit Corrections or Updates. | risk of COVID-19 case, 73.6% lower, RR 0.26, p < 0.001, treatment 11 of 389 (2.8%), control 52 of 486 (10.7%). |
References
1.Afsar et al., SSRN, Ivermectin Use Associated with Reduced Duration of COVID-19 Febrile Illness in a Community Setting, https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3734478.
2.Ahmed et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2020.11.191, A five day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness, https://www.sciencedirect.com/science/article/pii/S1201971220325066.
3.Alam et al., European Journal ofMedical and Health Sciences, doi:10.24018/ejmed.2020.2.6.599, Ivermectin as Pre-exposure Prophylaxis for COVID-19 among Healthcare Providers in a Selected Tertiary Hospital in Dhaka – An Observational Study, https://ejmed.org/index.php/ejmed/article/view/599.
4.Altman, D., BMJ, doi:10.1136/bmj.d2304, How to obtain the P value from a confidence interval, https://www.bmj.com/content/343/bmj.d2304.
5.Altman (B) et al., BMJ, doi:10.1136/bmj.d2090, How to obtain the confidence interval from a P value, https://www.bmj.com/content/343/bmj.d2090.
6.Anglemyer et al., Cochrane Database of Systematic Reviews 2014, Issue 4, doi:10.1002/14651858.MR000034.pub2, Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials, https://www.cochranelibrary.com/cd..0.1002/14651858.MR000034.pub2/full.
7.Asghar et al., NCT04392713, Efficacy of Ivermectin in COVID-19, https://clinicaltrials.gov/ct2/show/NCT04392713.
8.Babalola et al., QJM: An International Journal of Medicine, doi:10.1093/qjmed/hcab035 (preprint 1/6), Ivermectin shows clinical benefits in mild to moderate COVID19: A randomised controlled double-blind, dose-response study in Lagos, https://academic.oup.com/qjmed/adv../doi/10.1093/qjmed/hcab035/6143037.
9.Behera et al., Research Square, doi:10.21203/rs.3.rs-208785/v1, Prophylactic role of ivermectin in SARS-CoV-2 infection among healthcare workers, https://www.researchsquare.com/article/rs-208785/v1.
10.Behera (B) et al., PLOS ONE, doi:10.1371/journal.pone.0247163 (preprint 11/3), Role of ivermectin in the prevention of SARS-CoV-2 infection among healthcare workers in India: A matched case-control study, https://journals.plos.org/plosone/..le?id=10.1371/journal.pone.0247163.
11.Beltran-Gonzalez et al., medRxiv, doi:2021.02.18.21252037, Efficacy and safety of Ivermectin and Hydroxychloroquine in patients with severe COVID-19. A randomized controlled trial, https://www.medrxiv.org/content/10.1101/2021.02.18.21252037v1.
12.Bernigaud et al., Annals of Dermatology and Venereology, doi:10.1016/j.annder.2020.09.231, Ivermectin benefit: from scabies to COVID-19, an example of serendipity, https://www.sciencedirect.com/science/article/pii/S015196382030627X.
13.Budhiraja et al., medRxiv, doi:10.1101/2020.11.16.20232223, Clinical Profile of First 1000 COVID-19 Cases Admitted at Tertiary Care Hospitals and the Correlates of their Mortality: An Indian Experience, https://www.medrxiv.org/content/10.1101/2020.11.16.20232223v1.
14.Bukhari et al., medRxiv, doi:10.1101/2021.02.02.21250840, Efficacy of Ivermectin in COVID-19 Patients with Mild to Moderate Disease, https://www.medrxiv.org/content/10.1101/2021.02.02.21250840v1.
15.Cadegiani et al., medRxiv, doi:10.1101/2020.10.31.20223883, Early COVID-19 Therapy with Azithromycin Plus Nitazoxanide, Ivermectin or Hydroxychloroquine in Outpatient Settings Significantly Reduced Symptoms Compared to Known Outcomes in Untreated Patients, https://www.medrxiv.org/content/10.1101/2020.10.31.20223883v1.
16.Camprubí et al., PLoS ONE, 15:11, doi:10.1371/journal.pone.0242184, Lack of efficacy of standard doses of ivermectin in severe COVID-19 patients, https://journals.plos.org/plosone/..le?id=10.1371/journal.pone.0242184.
17.Carvallo et al., medRxiv, doi:10.1101/2020.09.10.20191619, Safety and Efficacy of the combined use of ivermectin, dexamethasone, enoxaparin and aspirin against COVID-19, https://www.medrxiv.org/content/10.1101/2020.09.10.20191619v1.
18.Carvallo (B) et al., Journal of Biomedical Research and Clinical Investigation, doi:10.31546/2633-8653.1007, Study of the Efficacy and Safety of Topical Ivermectin + Iota-Carrageenan in the Prophylaxis against COVID-19 in Health Personnel, https://medicalpressopenaccess.com/upload/1605709669_1007.pdf.
19.Carvallo (C) et al., NCT04425850, Usefulness of Topic Ivermectin and Carrageenan to Prevent Contagion of Covid 19 (IVERCAR), https://clinicaltrials.gov/ct2/show/results/NCT04425850.
20.Chaccour et al., EClinicalMedicine, doi:10.1016/j.eclinm.2020.100720 (preprint 12/7), The effect of early treatment with ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: A pilot, double-blind, placebo-controlled, randomized clinical trial, https://www.sciencedirect.com/science/article/pii/S2589537020304648.
21.Chachar et al., International Journal of Sciences, 9:31-35, doi:10.18483/ijSci.2378, Effectiveness of Ivermectin in SARS-CoV-2/COVID-19 Patients, https://www.ijsciences.com/pub/article/2378.
22.Chala et al., NCT04701710, Prophylaxis Covid-19 in Healthcare Agents by Intensive Treatment With Ivermectin and Iota-carrageenan (Ivercar-Tuc), https://clinicaltrials.gov/ct2/show/NCT04701710.
23.Concato et al., NEJM, 342:1887-1892, doi:10.1056/NEJM200006223422507, https://www.nejm.org/doi/full/10.1056/nejm200006223422507.
24.Deaton et al., Social Science & Medicine, 210, doi:10.1016/j.socscimed.2017.12.005, Understanding and misunderstanding randomized controlled trials, https://www.sciencedirect.com/science/article/pii/S0277953617307359.
25.Deng, H., PyMeta, Python module for meta-analysis, http://www.pymeta.com/.
26.Elalfy et al., J. Med. Virol., doi:10.1002/jmv.26880, Effect of a combination of Nitazoxanide, Ribavirin and Ivermectin plus zinc supplement (MANS.NRIZ study) on the clearance of mild COVID-1, https://onlinelibrary.wiley.com/doi/10.1002/jmv.26880.
27.Elgazzar et al., Research Square, doi:10.21203/rs.3.rs-100956/v2, Efficacy and Safety of Ivermectin for Treatment and prophylaxis of COVID-19 Pandemic, https://www.researchsquare.com/article/rs-100956/v3.
28.Elgazzar (B) et al., Research Square, doi:10.21203/rs.3.rs-100956/v2, Efficacy and Safety of Ivermectin for Treatment and prophylaxis of COVID-19 Pandemic, https://www.researchsquare.com/article/rs-100956/v3.
29.Espitia-Hernandez et al., Biomedical Research, 31:5, Effects of Ivermectin-azithromycin-cholecalciferol combined therapy on COVID-19 infected patients: A proof of concept study, https://www.biomedres.info/biomedi..-proof-of-concept-study-14435.html.
30.Gorial et al., medRxiv, doi:10.1101/2020.07.07.20145979, Effectiveness of Ivermectin as add-on Therapy in COVID-19 Management (Pilot Trial), https://www.medrxiv.org/content/10.1101/2020.07.07.20145979v1.
31.Hashim et al., medRxiv, doi:10.1101/2020.10.26.20219345, Controlled randomized clinical trial on using Ivermectin with Doxycycline for treating COVID-19 patients in Baghdad, Iraq, https://www.medrxiv.org/content/10.1101/2020.10.26.20219345v1.
32.Hellwig et al., International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2020.106248, A COVID-19 Prophylaxis? Lower incidence associated with prophylactic administration of Ivermectin, https://www.sciencedirect.com/science/article/pii/S0924857920304684.
33.Hill et al., Research Square, doi:10.21203/rs.3.rs-148845/v1, Meta-analysis of randomized trials of ivermectin to treat SARS-CoV-2 infection, https://www.researchsquare.com/article/rs-148845/v1.
34.Khan et al., Archivos de Bronconeumología, doi:10.1016/j.arbres.2020.08.007, Ivermectin treatment may improve the prognosis of patients with COVID-19, https://www.archbronconeumol.org/e..ognosis-articulo-S030028962030288X.
35.Kirti et al., medRxiv, doi:10.1101/2021.01.05.21249310, Ivermectin as a potential treatment for mild to moderate COVID-19: A double blind randomized placebo-controlled trial, https://www.medrxiv.org/content/10.1101/2021.01.05.21249310v1.
36.Kory et al., Feb 11, 2021, FLCCC Weekly Update – I-MASK+ and MATH+ Protocols, https://www.youtube.com/watch?v=xy85K1hENn4&t=1829s.
37.Kory (B) et al., FLCCC Alliance, Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19, https://covid19criticalcare.com/wp..axis-and-treatment-of-COVID-19.pdf.
38.Krolewiecki et al., SSRN, Antiviral Effect of High-Dose Ivermectin in Adults with COVID-19: A Pilot Randomised, Controlled, Open Label, Multicentre Trial, https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3714649.
39.Lawrie et al., Preprint, Ivermectin reduces the risk of death from COVID-19 – a rapid review and meta-analysis in support of the recommendation of the Front Line COVID-19 Critical Care Alliance, https://b3d2650e-e929-4448-a527-4e..b655bd21b1448ba6cf1f4c59f0d73d.pdf.
40.Lee et al., Arch Intern Med., 2011, 171:1, 18-22, doi:10.1001/archinternmed.2010.482, Analysis of Overall Level of Evidence Behind Infectious Diseases Society of America Practice Guidelines, https://jamanetwork.com/journals/j..nternalmedicine/fullarticle/226373.
41.Lima-Morales, Effectiveness of a multidrug therapy consisting of ivermectin, azithromycin, montelukast and acetylsalicylic acid to prevent hospitalization and death among ambulatory COVID-19 cases in Tlaxcala, Mexico, https://www.sciencedirect.com/science/article/pii/S1201971221001004.
42.Mahmud et al., Clinical Trial Results, NCT04523831, Clinical Trial of Ivermectin Plus Doxycycline for the Treatment of Confirmed Covid-19 Infection, https://clinicaltrials.gov/ct2/show/results/NCT04523831?view=results.
43.McLean et al., Open Forum Infect. Dis. September 2015, 2:3, doi:10.1093/ofid/ofv100, Impact of Late Oseltamivir Treatment on Influenza Symptoms in the Outpatient Setting: Results of a Randomized Trial, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4525010/.
44.Mohan et al., Ivermectin in mild and moderate COVID-19 (RIVET-COV): a randomized, placebo-controlled trial, https://www.researchsquare.com/article/rs-191648/v1.
45.Niaee et al., Research Square, doi:10.21203/rs.3.rs-109670/v1, Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: A randomized multi-center clinical trial, https://www.researchsquare.com/article/rs-109670/v1.
46.Nichol et al., Injury, 2010, doi: 10.1016/j.injury.2010.03.033, Challenging issues in randomised controlled trials, https://www.injuryjournal.com/article/S0020-1383(10)00233-0/fulltext.
47.Okumuş et al., NCT04646109, Evaluation of the Effectiveness and Safety of Adding Ivermectin to Treatment in Severe COVID-19 Patients, https://www.researchsquare.com/article/rs-224203/v1.
48.Podder et al., IMC J. Med. Science, 14:2, July 2020, Outcome of ivermectin treated mild to moderate COVID-19 cases: a single-centre, open-label, randomised controlled study, http://imcjms.com/registration/journal_abstract/353.
49.Raad et al., ChiCTR2000033627, In vivo use of ivermectin (IVR) for treatment for corona virus infected patients (COVID-19): a randomized controlled trial, http://www.chictr.org.cn/showprojen.aspx?proj=54707.
50.Rajter et al., Chest, doi:10.1016/j.chest.2020.10.009, Use of Ivermectin is Associated with Lower Mortality in Hospitalized Patients with COVID-19 (ICON study), https://www.sciencedirect.com/science/article/pii/S0012369220348984.
51.Rezai et al., IRCT20111224008507N3, Effectiveness of Ivermectin in the Treatment of Coronavirus Infection in Patients admitted to Educational Hospitals of Mazandaran in 2020, https://en.irct.ir/trial/49174.
52.Schwartz, E., Sheba Ivermectin Project, Ivermectin vs. placebo treatment in non-hospitalized patients with COVID-19 – A double blind, randomized controlled trial, https://vimeo.com/511687719.
53.Shouman et al., Journal of Clinical and Diagnostic Research, doi:10.7860/JCDR/2020/46795.0000, Use of Ivermectin as a Potential Chemoprophylaxis for COVID-19 in Egypt: A Randomised Clinical Trial, https://www.jcdr.net/articles/PDF/..Sh)_PF1(SY_OM)_PFA_(OM)_PN(KM).pdf.
54.Soto-Becerra et al., medRxiv, doi:10.1101/2020.10.06.20208066, Real-World Effectiveness of hydroxychloroquine, azithromycin, and ivermectin among hospitalized COVID-19 patients: Results of a target trial emulation using observational data from a nationwide Healthcare System in Peru, https://www.medrxiv.org/content/10.1101/2020.10.06.20208066v1.
55.Spoorthi et al., IAIM, 2020, 7:10, 177-182, Utility of Ivermectin and Doxycycline combination for the treatment of SARSCoV-2, http://iaimjournal.com/wp-content/..oads/2020/10/iaim_2020_0710_23.pdf.
56.Sweeting et al., Statistics in Medicine, doi:10.1002/sim.1761, What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data, https://onlinelibrary.wiley.com/doi/10.1002/sim.1761.
57.Treanor et al., JAMA, 2000, 283:8, 1016-1024, doi:10.1001/jama.283.8.1016, Efficacy and Safety of the Oral Neuraminidase Inhibitor Oseltamivir in Treating Acute Influenza: A Randomized Controlled Trial, https://jamanetwork.com/journals/jama/fullarticle/192425.
58.Vallejos et al., Trials, doi:10.1186/s13063-020-04813-1, Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19): a structured summary of a study protocol for a randomized controlled trial, https://trialsjournal.biomedcentra..rticles/10.1186/s13063-020-04813-1.
59.Zhang et al., JAMA, 80:19, 1690, doi:10.1001/jama.280.19.1690, What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes, https://jamanetwork.com/journals/jama/fullarticle/188182.
Related:
Ivermectin for Covid-19: Database of all Ivermectin COVID-19 studies – 35 trials and growing
Hydroxychloroquine & Covid-19: India and France, a cruel comparison
Use of ivermectin to treat coronavirus patients approved in Slovakia